
The value of validated cleanroom disinfection
A look at the Ecolab–STAXS® EN16615 Study
By Stefaan Vanhalle – R&D Manager STAXS®
Maintaining contamination control in cleanroom environments is a fundamental requirement for pharmaceutical and biotech manufacturers. As outlined in EU GMP Annex 1 (2022), the disinfection process must be validated by demonstrating both the suitability and effectiveness of disinfectants in the specific way they are used, including the products and surfaces involved. To address this, Ecolab and STAXS® jointly conducted a comprehensive study based on the EN 16615 standard, adapted to reflect real-world pharmaceutical cleanroom conditions.
The results provide strong scientific backing for cleanroom operators seeking to validate their disinfection processes efficiently and confidently.
Adapting EN 16615 for cleanroom conditions
The EN 16615 method, originally developed for general surface disinfection, was enhanced in this study to include cleanroom-relevant adaptations. These included the use of typical pharmaceutical facility surfaces such as stainless steel and vinyl flooring, as well as relevant microbial species like Staphylococcus epidermidis (a representative of skin flora) and Bacillus subtilis (a spore-forming organism). The study also evaluated DOTCH® cleanroom wipes and mops soaked with various Ecolab disinfectants, using a unidirectional mechanical wiping technique to reflect actual cleaning practices.
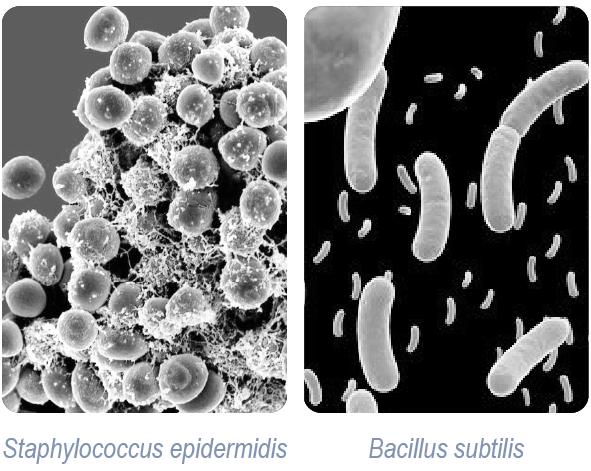
Proven dual-action efficacy
The results demonstrated a consistent and significant log reduction of microbial contaminants. Importantly, the study confirmed that microbial reduction is not only due to the biocidal action of the disinfectants but also to the high-quality design of DOTCH® mops and wipes, which physically capture and retain microorganisms through mechanical action. This dual-action performance ensures both chemical and physical removal of contaminants, which is essential in cleanroom protocols.

Customer benefits and regulatory confidence
| For customers, the benefits of this validated combination are substantial. Firstly, the study provides robust, externally generated data that can directly support validation programs under EU GMP Annex 15. This significantly reduces the internal resource burden typically required for method development and testing. Facilities can rely on the Ecolab–STAXS® data to justify their disinfection procedures, streamlining validation timelines and ensuring faster compliance with regulatory requirements. | Secondly, by validating the use of specific DOTCH® wipes and mops, in combination with Ecolab’s proven disinfectants, the study gives customers a high-confidence solution tailored to their controlled environments. The ability to demonstrate effectiveness across a variety of surface types and microbial challenges provides peace of mind and minimizes risk of contamination-related deviations or batch losses. |
Operational efficiency and product safety
Finally, the integration of mechanical and chemical efficacy means that customers benefit from an optimally designed cleaning system. This reduces the need for frequent re-cleaning, helps control residue build-up, and supports consistent environmental monitoring results, ultimately contributing to operational efficiency and product safety.

Conclusion
In conclusion, the Ecolab–STAXS® EN16615 study is more than a scientific validation; it is a practical tool that empowers cleanroom operators to meet GMP requirements with confidence, while simplifying processes and strengthening contamination control strategies.
Your step-by-step decision checklist
Got all the mopping techniques down? Use this handy checklist to consider all options when it comes to mops, and find out what works best for your controlled environment!References
-
EU GMP Annex 1 (2022), Manufacture of Sterile Medicinal Products
-
EU GMP Annex 15, Qualification and Validation
-
EN 16615:2015, Chemical Disinfectants and Antiseptics – Quantitative test method for surface disinfection
-
USP <1072>, Disinfectants and Antiseptics
