
Why you can't use just any mop in a controlled environment
Requirements for cleanroom use
By Stefaan Vanhalle – R&D Manager STAXS®
Cleaning procedures in cleanrooms demand consumables that don’t pose a contamination risk themselves. Even a seemingly simple tool like a floor mop needs to meet stringent quality criteria. With the publication of ISO 14644-18:2023, there is now, for the first time, an international guideline that systematically describes how to assess the suitability of consumables (such as mops), based on cleanliness attributes (particles, chemical residues, and biocontamination) and functional performance (absorption capacity, material compatibility, and sterilizability).
↓ Get your checklist below! ↓
Single-use vs. reusable mops
ISO 14644-18 distinguishes between single-use and reusable mops. Single-use mops are pre-validated for cleanliness and sterility. They offer consistent performance and reduce contamination risks, making them ideal for Grade A/B environments.
Reusable mops come with strict requirements. The entire laundering process needs to be validated to demonstrate that contaminants are effectively removed and that the mop’s integrity and cleanliness are maintained. Washing should be done in controlled conditions using approved detergents, and the number of reuse cycles has to be predefined, documented, and monitored.
The choice between single-use and reusable options should be risk-based and aligned with the cleanroom grade and facility procedures.
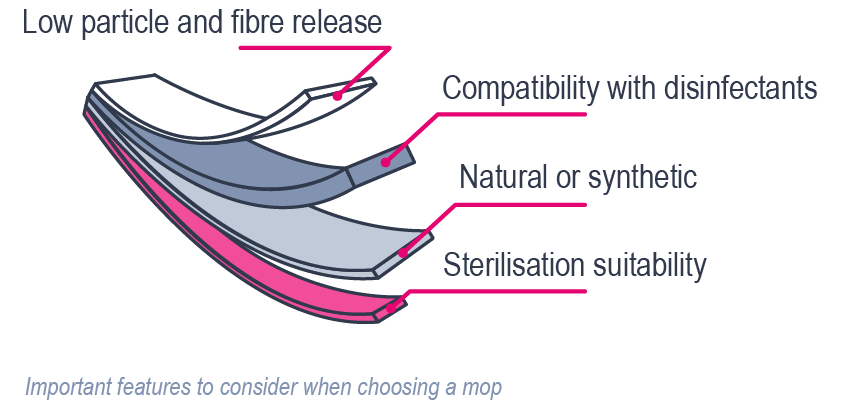
Particle and fiber release
A fundamental requirement for cleanroom mops is an extremely low release of particles and fibers. Any particles released from the mop material could possibly carry microorganisms. The Helmke drum test (IEST-RP-CC004.4) is widely used to evaluate this property. In this method, mop fabric is agitated inside a rotating drum, and a laser particle counter quantifies the emitted particles. The results are expressed in the number of particles per square centimeter.
Biocontamination and sterility
For aseptic zones, especially those classified as Grade A and B under EU GMP Annex 1, sterility of the floor mop at the point of use is mandatory. This means mop materials have to be compatible with sterilization techniques, like autoclaving or electron beam sterilization. The materials used need to withstand these processes without structural degradation. Additionally, to ensure aseptic transfer, mops have to be packaged in double or even triple-layer barrier systems.
Material selection and textile structure
The structure and composition of mop textiles significantly influence their performance and suitability. Flat-knitted fabrics made of 100% continuous filament polyester provide very low linting and good thermal resistance, but offer limited liquid retention.
Microfiber materials, typically blends of polyester and nylon, have a fine structure with high capillary action, making them effective for removing residual contamination.
Some mop designs incorporate an inner absorbent layer made of polyester, enclosed by a low-linting outer fabric. This multilayer structure combines good absorption capacity with uniform fluid distribution. These polyester-filled mops offer a balance between absorption and cleanliness.
Natural vs. synthetic fibers
An important consideration in cleanroom mop selection is the choice between natural and synthetic fibers.
| Natural fibers such as viscose offer several advantages: they are highly absorbent, cost-effective, and particularly well-suited for quick uptake and release of cleaning fluids. These properties make them an attractive option for a variety of controlled environments. | Synthetic fibers, particularly polyester and nylon, are the standard in more demanding environments. They offer exceptionally low particle emission, can be sterilized reliably, and are chemically inert. Continuous filament polyester, in particular, is preferred in ISO Class 4 to 6 cleanrooms and GMP Grade A/B areas. |
Although synthetic fibers are derived from fossil resources, they provide the consistency, durability, and sterilizability often required in controlled environments. Their environmental impact should be assessed on a case-by-case basis, considering the full lifecycle. Microfiber variants of synthetic materials also offer enhanced cleaning performance.
The choice between natural and synthetic materials should be based on a documented risk assessment, taking into account the cleanroom classification, process requirements, and contamination control objectives. When properly selected and applied, both fiber types can play an effective role in cleanroom cleaning strategies.

Compatibility with cleaning and disinfection agents
Another critical requirement is the compatibility of mop materials with common cleanroom disinfectants. Mops need to withstand exposure to agents such as 70% isopropyl alcohol, hydrogen peroxide, peracetic acid, and quaternary ammonium compounds without degrading, discoloring, or releasing additional contaminants. Chemical inertness is therefore essential to ensure both cleanliness and longevity in cleanroom operations.
Conclusion
Another critical requirement is the compatibility of mop materials with common cleanroom disinfectants. Mops need to withstand exposure to agents such as 70% isopropyl alcohol, hydrogen peroxide, peracetic acid, and quaternary ammonium compounds without degrading, discoloring, or releasing additional contaminants. Chemical inertness is therefore essential to ensure both cleanliness and longevity in cleanroom operations.
Your step-by-step decision checklist
Grab this easy to use list to guide you through the decision making process, so you can consider all options and find out what works best for your controlled environment!References
- ISO 14644-18:2023 – Cleanrooms and associated controlled environments – Part 18: Assessment of suitability for use of consumables
- EU GMP Annex 1 (2022) – Manufacture of sterile medicinal products (effective as of August 25, 2023)
- IEST-RP-CC004.4 – Evaluating Wiping Materials Used in Cleanrooms and Other Controlled Environments
- Whyte, W. (2010). Cleanroom Design (2nd ed.). CRC Press
- O'Neill, M.A. et al. (2021). Evaluation of microfiber mops for cleanroom use. Journal of Controlled Environments, 29(2), 13–20
